Unleash Your Imagination: Connect with Tumblr's Best!
Bond - Blog Posts

How do you decide on a Bond quote? It seems like a fools errand, there are too many famous quotes, so I'll try to go one level deeper: "Be polite, be courteous, show professionalism, and have a plan to kill everyone in the room." -James Bond (and James Mattis) "I don't stop when I'm tired, I stop when I'm done." -James Bond A found picture from the Rotary/Rotaract's, Secret Agent & Bond Girl Charity Soiree & Fundraiser. After two years working with the Rotaract club helping young people learn about financial literacy, I was flattered to receive an invite to the event. Even more so when the person who invited me held ticket 007 just for me. A night of music, secret missions, code words, drinks, and even an attempted assassination made helping the charity more fun than work. Philanthropy is always worth it. #finance #financialplanning #entrepreneur #workhardplayhard #hustle #quotes #blessed #Calgary #yyc #thankful #legacy #leader #goals #bond #007 #secretagent #beyourownbanker #betterlatethannever #sharecalgary #calgary360 #shareyyc #rotary #rotaract (at Sky 360 Restaurant)

She always has been. Always will be.
Everything and everyone else is only to supplement what we have together.

It’s about much more than just sex

The D/s dynamic is often very intense and serious.
Don't forget to save a place for fun and laughter.
Stacy has always been and will always be the center of my world. The rest of you are icing on the cake

Marriage and Parenting - A Beautiful Journey
Marriage and parenting are two of life’s most profound and transformative experiences. The synergy between these two life stages can create a harmonious cycle of growth, challenges, and joy. As partners evolve into parents, their relationship faces new dimensions and responsibilities. This article delves into the intricate dance between marriage and parenting, highlighting the rewards and…

View On WordPress
.꙳・•・❥・♡꙳𓂃𓂂*・❥・꙳•.
It's not just a matter of lust between two people.. There is an invisible bond that connects minds, souls, hearts, where there is no distance that would keep them apart. And there, between the mind and the body, they created a intimate space in which a common heart beat. And when you put your heart into it, there is nothing you can do. You are part of a common organism. Forever... M. •・꙳.𝓜𝔂 𝓽𝓱𝓸𝓾𝓰𝓱𝓽𝓼・꙳•.

.・♡꙳𓂃𓂂*・❥・꙳•. .・♡꙳𓂃𓂂・꙳•.

.・•.✦𓂃☆.•.♡.•・❥•.
It's not just a matter of lust between two people.. There is an invisible bond that connects minds, souls, hearts, where there is no distance that would keep them apart. And there, between the mind and the body, they created a intimate space in which a common heart beat. And when you put your heart into it, there is nothing you can do. You are part of a common organism. Forever... M. ・꙳𓂂✦𓂃・•.

One who can read your words & understands true meaning of each. Such person has the right to read your life like an "Author".
andra människor har också använt nanomaskiner i sin sci-fi. Många bond filmer släpptes efter mgs 1-3, majoriteten om inte ALLA dan Craig bond filmerna var post mgs 3. Anann sak, inte för att vara pedant men det hade räckt med att du skrev mgs 3, mgs 1 kan inte släppas innan mgs 3. Det är bara den senaste som inkluderar nanobots, jag skulle säga att no time to die är den enda som är MGS-ig i de nya bond filmerna, de andra inkl. kasino royale, quantum of solace, leap of faith, specter, är ganska distinkta. Skulle säga att det endast är no time to die som är MGS-ig. nanomaskinerna mannen de finns andra källor som använder dem också, kom igen läs på nästa gång lite de här är ett allmänt forum
I think every bond movie made after mgs 1-3 owes a debt to kojima. there was a nanomachines
Covalent Bonds: Sharing Is Caring!
Welcome to my second out of three posts on bonding - ionic, covalent and metallic. This post also covers the coordinate/ dative bond which I can’t remember if I’ve covered before. Only one more of this series left! Find the others here.
Covalent bonding involves one or more shared pairs of electrons between two atoms. These can be found in simple molecular elements and compounds like CO2 , macromolecular structures like diamond and molecular ions such as ammonium. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms – double and triple bonds represented with two and three lines respectively.
Dot and cross diagrams show the arrangement of electrons in covalent bonds. They use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.

The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds. For example, if an atom wants to make three covalent bonds but has a full 3s2 shell and a 3p1 shell, it can promote one of its 3s2 electrons so that an electron from the other atoms can fill the 3s shell and pair with the new 3p2 shell.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.

A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.

This is an example of a coordinate (sometimes called dative) bond between ammonia and a H+ ion which has an empty orbital. The lone pair on the ammonia overlaps with this H+ ion and donates its electrons. Both electrons come from the ammonia’s lone pair so it is a coordinate bond. This is demonstrated with an arrow. The diagram is missing an overall charge of + on the ammonium ion it produces. Coordinate bonds act the same as covalent bonds.
Once you have your covalent bonds, you need to know about covalent substances and their properties. There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these. Iodine (shown below) has a regular arrangement which makes it a crystalline substance and water, as ice, has a crystalline structure as well.

The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
The other kind of covalent substance you need to know is macromolecular. This includes giant covalent structures such as diamond or graphite, which are allotropes of carbon. Non-metallic elements and compounds usually form these crystalline structures with a regular arrangement of atoms.
Allotropes are different forms of the same element in the same physical state.
Diamond is the hardest naturally occurring substance on earth therefore is good for cutting glass and drilling and mining. It has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
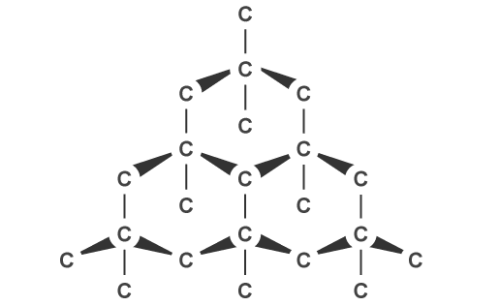
Graphite, on the other hand, can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.

Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
SUMMARY
Covalent bonding involves one or more shared pairs of electrons between two atoms. Covalent bonds mostly occur between non-metals but sometimes metals can form covalent bonds.
Single covalent bonds share just one pair of electrons. Double covalent bonds share two. Triple covalent bonds share three.
Each atom usually provides one electron – unpaired in the orbital – in the bond. The number of unpaired electrons in an atom usually shows how many bonds it can make but sometimes atoms promote electrons to fit in more. Covalent bonds are represented with lines between the atoms.
Dot and cross diagrams use dots and crosses to demonstrate that the electrons come from different places and often only the outer shell is shown.
The simple explanation as to how atoms form covalent bonds is that one unpaired electron in the orbital of one atom overlaps with one in another atom. Sometimes atoms promote electrons in the same energy level to form more covalent bonds.
Sometimes promotion does not occur and that means different compounds can be made such as PCl3 or PCl5.
A lone pair of electrons is a pair of electrons from the same energy sub-level uninvolved in bonding. Sometimes these can form something called a coordinate bond, which contains a shared pair of electrons where both come from one atom. The lone pair of electrons is “donated” into the empty orbital of another atom to form a coordinate bond.
The formation of ammonium is an example of this.
There are two types of covalent substance: simple covalent (molecular) and macromolecular (giant covalent).
Molecular simply means that the formula for the compound or element describes exactly how many atoms are in one molecule, e.g. H2O. Molecular covalent crystalline substances usually exist as single molecules such as iodine or oxygen. They are usually gases or liquids at room temperature but can be low melting point solids.
Solid molecular covalent solids are crystalline so can be called molecular covalent crystals. Iodine and ice are examples of these.
The properties of these crystals are that they have low melting points, are very brittle due to the lack of strong bonds holding them together and also do not conduct electricity since no ions are present.
Giant covalent structures such as diamond or graphite are allotropes of carbon. Allotropes are different forms of the same element in the same physical state.
Diamond has a high melting point due to the many covalent bonds which require a lot of energy to break. Each carbon has four of these bonds joining it to four others in a tetrahedral arrangement with a bond angle of 109.5 degrees and it does not conduct electricity or heat because there are no ions free to move.
Graphite can conduct electricity. This is because it has delocalised electrons between the layers which move and carry charge. Carbon atoms within the structure are only bonded to three others in a hexagonal arrangement with a bond angle of 120 degrees. Since only three of carbon’s unpaired electrons are used in bonding, the fourth becomes delocalised and moves between the layers of graphite causing weak attractions, explaining why it can conduct electricity.
Graphite’s layered structure and the weak forces of attractions between it make it a good lubricant and ideal for pencil lead because the layers can slide over each other. The attractions can be broken easily but the covalent bonds within the layers give graphite a high melting point due to the amount of energy needed to break them.
Happy studying!
The Name’s Bond ... Ionic Bond.
This is the first in my short series of the three main types of bond - ionic, metallic and covalent. In this, you’ll learn about the properties of the compounds, which atoms they’re found between and how the bonds are formed. Enjoy!
When electrons are transferred from a metal to a non-metal, an ionic compound is formed. Metals usually lose electrons and non-metals usually gain them to get to a noble gas configuration. Transition metals do not always achieve this.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. Make sure you know that the transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
You need to know how to explain how atoms react with other atoms and for this the electron configurations are needed. You can use dot and cross diagrams for this.

Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid. For example, NaCl has a 6:6 arrangement - each Na+ ion is surrounded by 6 Cl- and vice versa.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating. Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions. The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.

Look at this diagram. It shows how atomic radius decreases across a period regularly. This is not the case with the ions. Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. For negative ions, they become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.

Ionic compounds are usually soluble in water. This is because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other. Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
SUMMARY
When electrons are transferred from a metal to a non-metal, an ionic compound is formed.
Charged particles that have either lost or gained electrons are called ions and are no longer neutral - metal atoms lose electrons to become positive ions (cations) whereas non-metals gain electrons to become negative ions (anions).
The formation of these ions is usually shown using electron configurations. The transfer of electrons is not the bond but how the ions are formed.
An ionic bond is the electrostatic attraction between oppositely charged ions.
Ionic solids hold ions in 3D structures called ionic lattices. A lattice is a repeating 3D pattern in a crystalline solid.
Ionic solids have many strong electrostatic attractions between their ions. The crystalline shape can be decrepitated (cracked) on heating.
Ionic Lattices have high melting and boiling points since they need more energy to break because atoms are held together by lots of strong electrostatic attractions between positive and negative ions.
The boiling point of an ionic compound depends on the size of the atomic radius and the charge of the ion. The smaller the ion and the higher the charge, the stronger attraction.
Positive ions are usually smaller than the atoms they came from because metal atoms lose electrons meaning the nuclear charge increases which draws the electrons closer to the nucleus. Negative ions become larger because repulsion between electrons moves them further away - nuclear charge also decreases as more electrons to the same number of protons.
Ionic substances can conduct electricity through the movement of charged particles when molten or dissolved (aqueous). This is because when they are like this, electrons are free to move and carry a charge. Ionic solids cannot conduct electricity.
Ionic compounds are usually soluble in water because the polar water molecules cluster around ions which have broken off the lattice and so separate them from each other.
Some substances like aluminium oxide have too strong electrostatic attractions so water cannot break up the lattice - it is insoluble in water.
Molecular ions such as sulfate, nitrate, ammonium or carbonate can exist within ionic compounds. These compounds may have covalent bonds within the ions but overall they are ionic and exhibit thee properties described above.
WHOSE READY FOR SPYXFAMILY????

Ignore the poorly drawn shadow the hedgehog in the corner lol
please don’t steal, repost, and claim my artwork. Likes and comments are great! Reblogs too
✨BOND SPELL🕯
*** This spell is meant for increasing the bond of a previously established relationship. This spell won’t create a relationship that was not previously there.***
CHANT:
Like a bee to a flower, we need to be lovers, one cannot live without the other, and should it be found that we are apart, we’ll be connected at the core of our hearts. For I am your one and you are to mine, forever and always, together in time.
WHAT YOU’LL NEED:
1. A purple candle 2. A gold thread/string
WHAT YOU’LL NEED TO DO:
1. Light the purple candle. 2. The gold thread will be a representation of your bond. Wrap it around your candle and tie a knot ONLY ONCE while repeating the chant. 3. Keep this candle at your bedside or somewhere in the room that you share with your partner. You can keep the candle lit as long as you would like but you can blow out the candle once the knot has been tied. 4. Every night, simply light the candle and retighten the knot. This represents strengthening the bond of your relationship continuously. Because relationships are a continuous effort, they must be continuously strengthened. You may retighten throughout the day if necessary. Repeat the chant while doing this as often as you can (kind of like singing during any task). ***OPTIONAL: You may engrave a sigil of Strength into the side of the candle***
Blessed be.
![[Well, That’s A Bloody Big Ship.]](https://64.media.tumblr.com/666acf2ea4cd9edd7dbf0affefaedcc2/tumblr_o0epnquuRo1si9cp1o1_500.jpg)
[Well, that’s a bloody big ship.]
HAPPY BIRTHDAY @kittendough!! Alte Schachtel Some 00Q, just for you! Hope you have a wonderful day/year/life! :DD

I made this in honor of "Sean C Saturday" or just #scs which I also made up. Betta check ya shelf before ya wreck ya shelf
"Talk Mr. Bond? I will be sitting on your face. I expect you will be too busy to talk."


If Anya was into Star Wars this would happen

Recently had several conversations with folks about the history and current state of the Bond franchise. I'm a pretty huge fan of the series as a whole, but could be a whole lot happier about the current choices being made about the (historically) suave and cunning spy in the suit.
I've just seen Spectre
I love Ben Whishaw as Q! Also I heard that Daniel Criag chose to do another Bond movie!! I hope it is true I also think that Lea Seydoux was a perfect bond girl after Eva Green to be honest
Can I request William James Moriarty x reader making out and reader was so loud that everyone heard them so when they got down Moran and Bond were teasing reader and she is so emotional so she started crying and ran from room and Louis, William, Albert and Jack wanted to kill these two but they went straight to apologize to reader
The right Decision
William James Moriarty x female!reader Request
Word count:1.2k
Warnings:making out, emotional reader
Summary: You and William were supposed to join the others for tea but you have other plans..
Masterlist

A few weeks ago, I wouldn't even have dared to think about what was happening right now. A few weeks ago, William was nothing more than a close friend, just like the rest of his family. But now, it was different. Since I confessed my love to him, he never seemed to be more than ten meters away from me. To be honest, I was surprised that he even wanted to persue a relationship with me, considering the plans he had.
But he did nevertheless.
And that's exactly how we ended up here. Huddled up in William's bedroom, with his hands all over my body, while we were actually supposed to join the others downstairs for tea. I could smell the sweet scent of Louis'selfmade green tea all the way up to where we were sitting on William's bed.
"Will?", I mumbled, as he softly kissed the sensitive skin behind my ear.
"Will, we should join the others", I tried to reason with him, but William only whined and raised his head to look at me.
"Five more minutes", he whispered and softly caressed my cheek with his tender fingers.
"This is so unlike you, Will", I mumbled, while he pulled me into his lap.
"It's because you make me feel a whole new emotion", He mumbled, his crimson eyes fixated on mine.
I felt a blush creep up at my neck. William was so different now. While he was already caring and friendly before, he now seemed like he wanted to protect me at all costs. His soft touches telling me that he would never dare to let anything happen to me.
"Is it Love that you feel?", I asked, as his hands started to push up my skirt, his palms resting on my thighs while I pulled his face back to mine.
"That and so much more", he confessed. A sigh escaped me, as he pressed his lips against mine in a tender, yet passionate kiss. I felt how he pulled me impossibly closer. And it almost felt like this moment could last forever, like he wouldn't die in a few months. It felt like he would throw all that away and just stay with me forever.
But I knew that he would never do that. He had worked his entire life for this and he wouldn't give it up for me.
"I know, what you are thinking about, my love", William said, as we parted,"You are thinking about my death, aren't you"
I only nodded. I knew lying to him was worthless. I watched as a soft smile appeared on his face.
"You know", he mumbled, placing a soft kiss on my forehead,"If I would've met you earlier, I might've stopped persuing my goal"
My eyes widened upon hearing his words:"Really?!"
He only nodded:"I would've made sure that protecting you is the only Goal I have"
"Oh, Will", I sighed pulling him back towards my lips. I knew that this meant much more than just saying 'I love you'.
And now as he kissed me so passionately and touched me so gently, I knew that I wanted to spend the rest of my life with him. And as moan after moan escaped my lips, I knew that I wanted nothing more that for him to live. But I knew that I couldn't change his mind. I would try anyways though...

"Look, who finally made it to tea", I heard Moran say, as William and me finally made our way downstairs to the others. I could see that him and Albert were playing a game of chess. Albert was about to win though.
"Sorry, that we're late", William smiled and took my hand, pulling me into his side,"We were just enyjoying some time alone together."
"Of course you were", Bond smiled, a cup of tea still in his hand, as he sat down between Louis and Fred on the sofa.
I already felt my cheeks blush, as William guided me into the room. Suddenly everything felt so wrong. I almost regretted giving in to William's request to stay in his room a little while longer. The only thing grounding me right now, was his firm grip around my waist.
"Y/N, tell me how is William doing as your lover so far. He almost seems to innocent for a parter", Moran grinned, his dark eyes fixated on mine. Usually, his flirty behavior didn't bother me but right now it all seemed too overhelming.
"We're doing fine, Colonel", William smiled politely, as he took a step forward to reach for the two ramaining cups on the table.
"Oh, we could certainly hear that", Moran grinned and mindlessly moved a chess piece in front of him.
And that little comment was enough. I didn't know what had gotten into me, but my shoulders slumbed and I felt tears burning in my eyes.
"Excuse me", I mubled, before quickly making my way back upstairs. I didn't want them to see my like this. So vulnarable. It was enough, if William had to see this side of me.
I quickly made my way back into William's room. I threw the door shut behind me and immediately retreated onto his balcony. As the fresh air hit my face and the soft rays of the afternoon sun hit my face, I could already feel myself calm down again. Today was probably just an emotional day. With William's confession earlier, I felt alll over the place.
A quiet,"God damn it", left my lips as I cursed the fact that I couldn't control my feelings at times like these. Especially because I wasn't the only one who felt like this. With the great finale of William's plan coming closer and closer, every single one of us was on edge. And I had to be the one who needed taking care of again, as always. That thought almost made me want to cry again and I fiercely gripped the balustrade of the balcony.
"My love?"
My eyes widened, as I heard William's voice behind me. I turned around and saw him standing in the middle of his room. The rest of the group seemingly wanted to hide behind his frame.
"I'm sorry, Y/N", Moran said and immediately made his way over to me. He looked at me ashamed and took my hand, falling to his knees,"I know it's not easy for you. I shouldn't have said, what I said."
"Its fine, Moran", I assured him and moitioned for him to stand up again. What he didn't expect was, that I pulled him into a firm hug.
"We're all on edge, so no worries", I explained and looked at the others with a smile,"I trust William and I know his plan will work out. For all of us."
"Thank you, my beloved",William said and bowed slightly. The others followed his example.
"Now come on", I said, striding towards him and taking his hand,"Let's drink a cup of tea, before it gets dark outside"
"It would be my pleasure", Will smiled.
Together with the others, we walked back to the living room and I couldn't say that I regretted it, because I got to see Moran despair in a game of chess against William. So, in the end, I knew that pursuing this relationship was the right decision.


